Back
Enabling The 10X Data Manager
Data managers are the unsung heroes of the clinical trial process. They build a foundational level of hygiene into the study database, enabling all downstream analysis. They own the delivery of that final clean database, managing the challenges of protocol variability, site performance, and cross-functional responsibilities throughout the study. It's a thankless job candidly, and as studies get more complex and data volumes increase, the burden on data managers will only continue to grow.
In software engineering, the term “10X engineer” refers to engineers who are incredibly efficient at the foundational practices of coding, can see the big picture, and can tackle problems with ease. Our team at Oovacha thinks a lot about how we can help infuse these capabilities within the clinical research industry and equip data managers with better tools, so that we can enable an entire industry of 10x Data Managers.
Shift from Manual Work to Intelligent Supervision
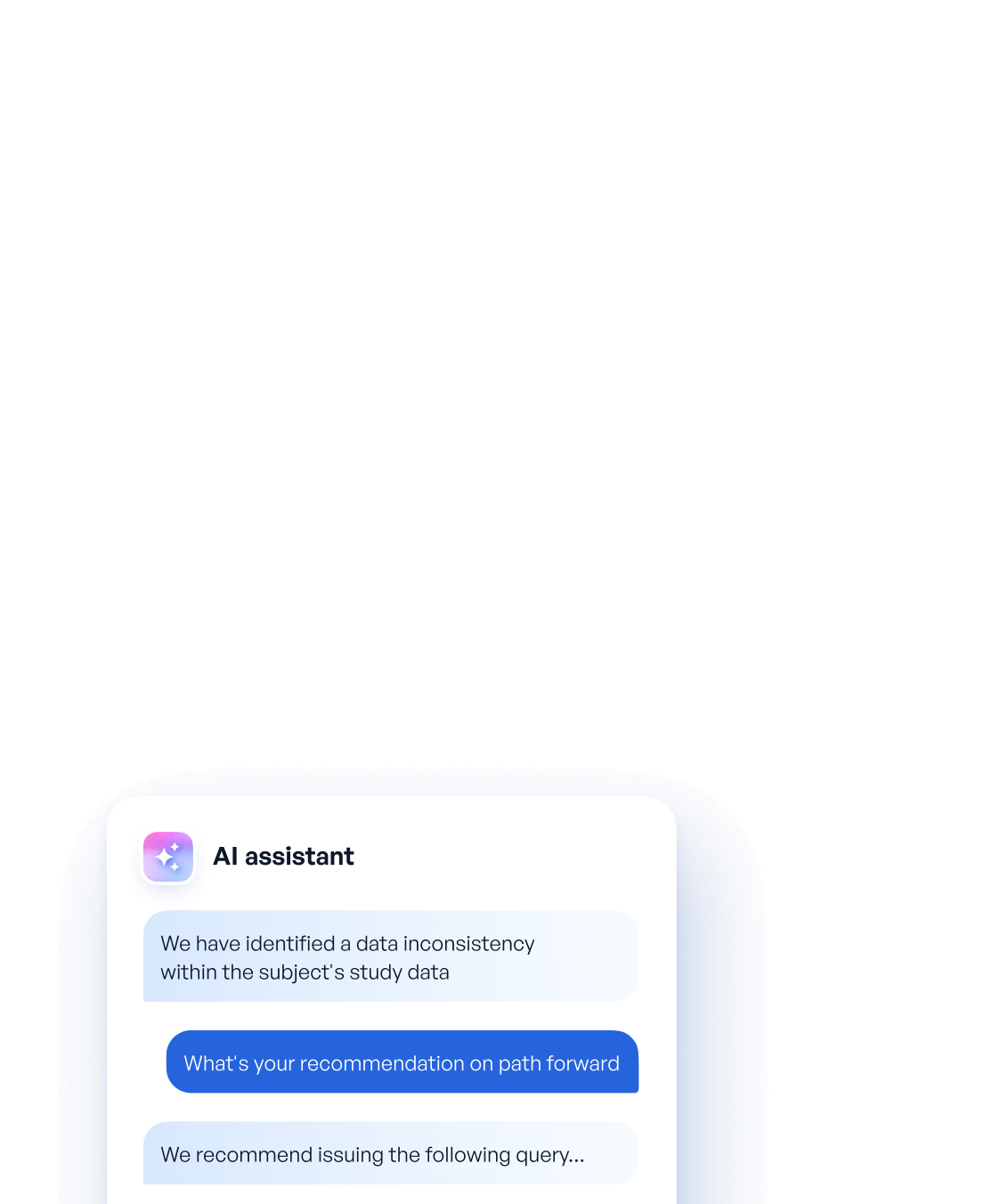
Today data managers are forced to deal with a (still) heavily manual data cleaning process. One characterized by endless SAS listings, raw data investigation and query writing. Our platform Reveal equips data managers with AI assistants that take on much of this manual burden and decrease average time required to issue a query by over 50%.
The 10X data manager wont be bogged down in manual review of listings and query writing. Instead, they’ll be supervising AI assistants, approving recommendations and spending their time addressing more complex trial issues.
From Data Management to Data Science
Over the past few years, the term “Clinical Data Science” has become an increasingly popular term, representing a discipline focused on uncovering insights and resolving study risks before they spiral out of control. Clinical research teams are increasingly looking to uncover:
- Longitudinal insights – patient level trajectories that highlight safety issues or implausible trends
- Consistency across different domains (e.g., labs, AEs, conmeds) – to verify the accuracy of study data
- Site and study level trends – to identify systemic deviations that might necessitate re-training or study-level re-design
These are the insights that drive faster database locks and more reliable study outcomes. As data managers delegate the more mundane work to AI assistants, data managers can allocate their time to analyzing and resolving these more complex trial issues. Data managers can finally embrace this evolution of clinical data science.
Why the 10X Data Manager Matters
The promise of the 10x Data Manager is straightforward: greater impact with improved efficiency. With less manual burden on their plate, Data Managers can focus on strategic analysis and oversight instead of repetitive tasks. And as the backbone of the entire trial process, the whole trial benefits:
Improved oversight over site performance and trial design
Smoother delivery of key trial artifacts
The concept of the 10X data manager won’t be achieved with a single individual, it requires an organizational shift. It involves reinventing how data management teams operate and investing in the technology needed to enable these efficiencies.