Back
It’s Time for Clinical Trial Data Review to Evolve
Clean, reliable data is the foundation of every trial.
Before any real insights can be gleaned from a trials’ results, all collected data needs to be cleaned, verified and signed off on. But while the drug modalities and trial designs have seen incredible innovation over the last few decades, the way the clinical research industry reviews and cleans clinical trial data has not changed much.
Most studies today rely on batch-based query cycles. They involve monthly data freezes, static error reports and several weeks before queries reach sites. For data managers and clinical research teams, this means weeks of delay (nearly 45 days before a query reaches a site and 2-3 months before data is considered “clean”), stale insights, and errors that surface long after they should have been caught.
The Batch Review Status Quo
In most trials, the process works like this:
- Data Freeze: EDC study data is “frozen” before a review cycle begins (every 2-4 weeks)
- Listings Prepared: Pre-configured SAS listings are prepared based on the frozen study data
- Error Reports Generated: SAS programmers generate and distribute error reports to data managers, medical monitors, and clinical operations.
- Reports Reviewed and Queries Issues: Teams review the reports and issue queries in the EDC over the following 2-4 weeks
For data managers, this process involves weeks of manual investigation. And for the site, it means getting queries about data they entered weeks ago, sometimes even when the patient has already moved on to their next visit.
Why this is bad?
Until now, the industry has had no choice but to rely on freezing the EDC system and batch-based review. If a data manager tried to review and analyze all the EDC data before the next update is made, it simply wouldn’t be humanly possible. But the status quo of batch-based review unfortunately leads to real (and costly) problems:
- Trial Milestone Delays - Queries raised weeks late may delay access to clean data needed for interim analyses, DMC reviews, or database lock. Even a week delay in DB lock can cost sponsors ~$250K in added expense
- Stale Data for Review - By the time trial teams sit down with reports, much of the data is already outdated, making it harder to act with confidence
- Site Burden and Frustration - Sites struggle to respond to queries about old visits, increasing cycle times and challenging site relationships
- Inefficient use of Data Management Talent - Highly trained data managers spend hours reconciling listings and drafting repetitive queries instead of focusing on oversight and decision-making
- Regulatory Risk - Under ICH E6(R3), regulators now expect proactive sponsor oversight. A delayed batch process makes it harder to demonstrate compliance
Opportunity to Move from Batch to Real-Time Review
Until now, the industry has had no choice but to rely on freezing the EDC system and batch-based review. If a data manager tried to review and analyze all the EDC data before the next update is made, it simply wouldn’t be humanly possible. But the status quo of batch-based review unfortunately leads to real (and costly) problems:
The limitations of technology had forced the industry to rely on batch-based review. But now that we can create AI Assistants (a.k.a AI agents) that can harness the immense power of LLMs and analyze trial data around the clock, data review can now be performed continuously and in real-time.
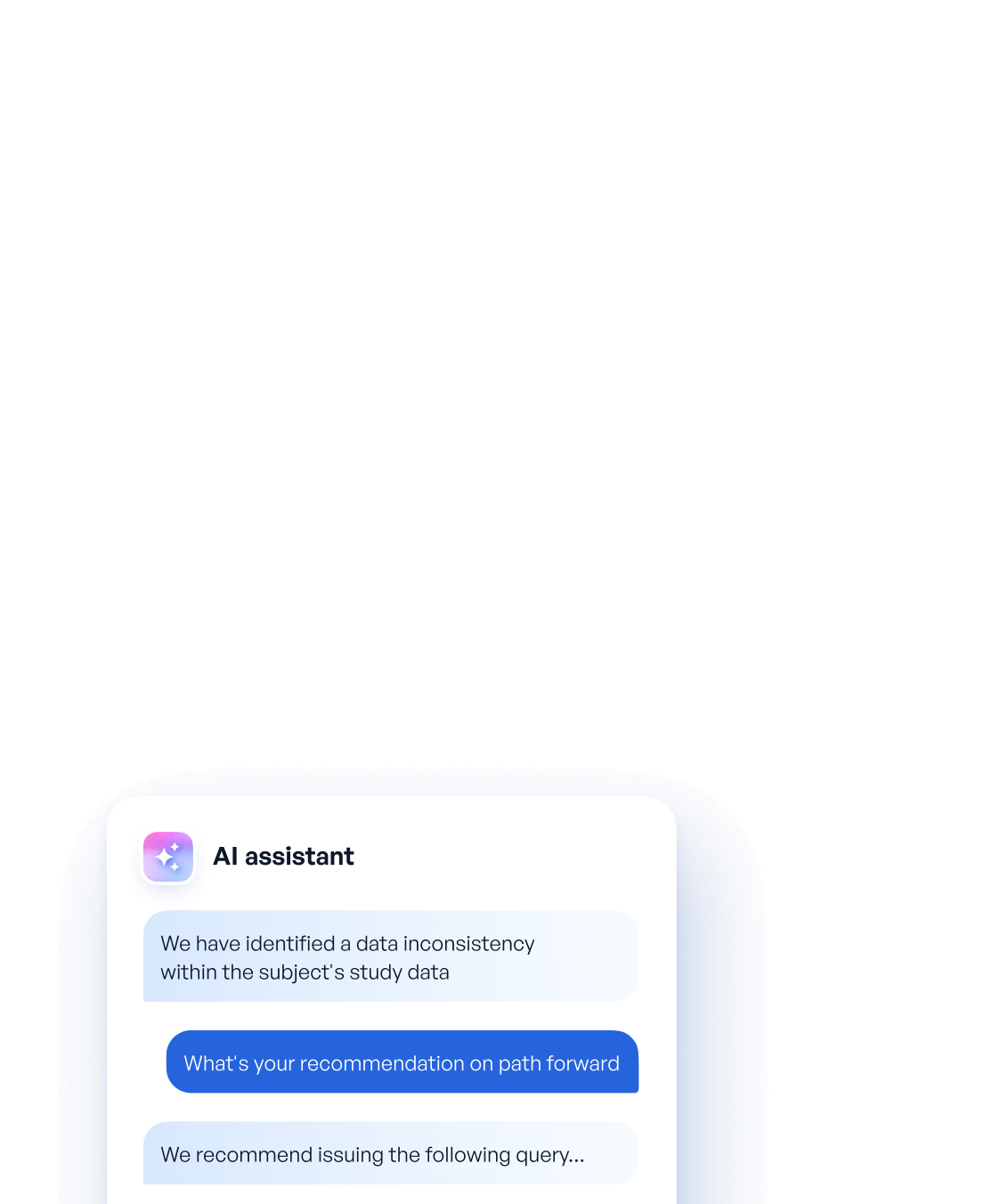
Let me explain how our platform Reveal uses AI assistants to create a live feed of data quality issues so that data-review can be performed in real time:
- First, updates to study data are analyzed daily
- Then, our assistants gather all updated data, and identify (using their powerful underlying large language models) any relevant data inconsistencies or discrepancies
- Once these discrepancies are found, our AI assistants will then recommend a path forward around resolution. If it makes sense to issue a query, then context-specific query messages are drafted and surfaced to data managers for their approval
In this new paradigm, data quality issues can be flagged within 24 hours of data entry and queries can be issued within 7 days. This represents an 80% decrease in the cycle time between data entry and query issuance. By compressing the cycle time, we can resolve issues quicker, analyze fresh data throughout a trial and avoid delays in trial timelines.
To Sum Up
Batch review has been the clinical research industry’s only solution to generate clean trial data. But now that we have the tech to eliminate the bottlenecks in the data review process, it’s time for the industry to level up. Let’s give data managers the tools they need to deliver higher quality data and faster turnarounds, so we can put the days of keeping study teams in the dark behind us.